As the coronavirus continues its grip on the world, scientists across the globe are working to find a viable vaccine. Several candidates are in the final stages of testing, but researchers warn it could still take years.

After researchers in China published the genome sequence of the coronavirus in January, scientists around the world set to work on a vaccine. The first candidate began human trials on March 16.
It’s now joined by nearly 200 others being tracked by the World Health Organization — 44 of which have entered human trials.
It normally takes years to test, produce and deploy vaccines. The fastest ever vaccine to be brought from clinical testing to market, the vaccine for mumps, took four years in the 1960s.
Scientists working on coronavirus vaccines are hoping to deliver one within 12 to 18 months. And the WHO hopes to roll out two billion doses of a vaccine by the end of 2021.
No coronavirus vaccines have yet been approved for general use internationally, but several candidates have reached the final stages of testing.
They are based on several different approaches, including active, inactivated, DNA, RNA/mRNA-based, virus vectors and protein subunits, and there are three test phases vaccines must pass before they are sent to regulatory authorities for approval.
Here’s an overview of the front-runners.
CoronaVac: Sinovac Biotech
CoronaVac, developed by Chinese firm Sinovac Biotech, is one such inactivated type of vaccine racing toward the finish line. Study results from Phase 3 trials, which are currently being carried out with tens of thousands of volunteers in Brazil, Turkey and Indonesia, are expected to be available in November. Although clinical trials are still underway, CoronaVac was approved for emergency use in China in late August as part of a programme to vaccinate high-risk groups, such as medical workers. Initial results from studies on macaque monkeys showed that the vaccine produced antibodies that neutralized 10 strains of SARS-CoV-2. Preprint results from Phase 2 human trials show it produced antibodies with no severe adverse reactions.
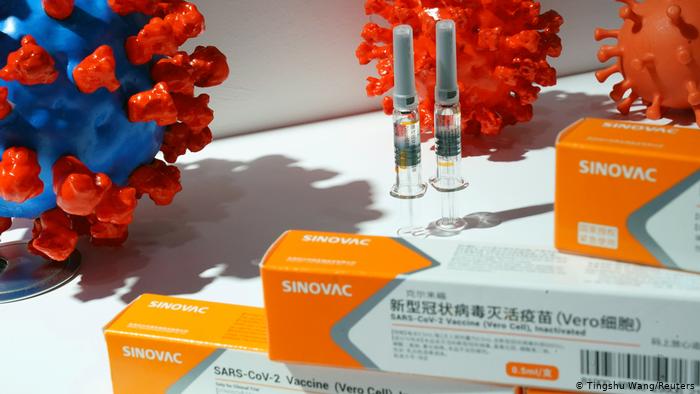
Brazil plans to use the Chinese-made Sinovac Biotech coronavirus vaccine as part of its national immunization programme
BNT162b2: Pfizer & BioNTech
BNT162b2 is a messenger RNA (mRNA) vaccine from American-German duo Pfizer and BioNTech. It’s currently in Phase 3 trials with 44,000 volunteers in multiple areas around the world with high rates of coronavirus transmission. Preliminary testing in the first two phases showed the vaccine produces antibodies and T-cell responses specific to the SARS-CoV-2 protein. Developers say they could know by the end of October if the vaccine works or not and have enough data to determine its safety by the end of November. No mRNA vaccine has ever been approved for an infectious disease — but proponents say it could be easier to produce than traditional vaccines.
mRNA-1273: Moderna
Another mRNA vaccine in the works, the mRNA-1273 from US-based Moderna, entered its third phase with 30,000 people in late July. Initial results from Phase 1 showed both young and elderly volunteers produced coronavirus antibodies and reactions from T-cells. In the trial, half of the volunteers receive a vaccine, while the other half get a placebo. Developers will be able to do a first analysis when 53 people in the entire volunteer group get symptomatic COVID-19 — the efficacy of the vaccine depends on whether there are significantly fewer vaccinated people than unvaccinated among the 53 cases.
Experts warn that vaccines need to be studied even after they are rolled out
Two of the most favored vaccine candidates — from AstraZeneca and Johnson & Johnson — have been paused while their developers investigate adverse reactions among participants.
ChAdOx1 nCoV-19: AstraZeneca & Oxford University
ChAdOx1 nCoV-19 is a viral vector vaccine in Phase 3 of trials, with developers aiming to recruit 50,000 volunteers. Initial results from the first two clinical phases showed the vaccine triggered a strong immune response, producing antibodies and T-cell responses in volunteers. The study was put on hold due to an unexplained illness in one UK participant, and has since resumed in Brazil, South Africa and the UK, although the US trial is yet to recommence.
Ad26.COV2-S: Johnson & Johnson
Johnson & Johnson, who are also producing an adenovector vaccine, recruiting around 60,000 people in various different countries, paused trials on October 12, saying an unexplained illness required an independent safety review. It is not uncommon for clinical trials to experience pauses, but it isn’t often that they are reported. Results from animal trials showed the vaccine produced “robust neutralizing” antibodies in macaques, and provided “complete or near complete protection” after a single dose.
Sputnik V: Gameleya National Center of Epidemiology and Microbiology
Russia’s Sputnik V, based on two adenovirus vectors, has also drawn widespread attention, after the Russian government approved it for general use on August 11 without completing Phase 3 trials. Results from the first two trials showed a strong immune response among the 76 participants.
Vaccine testing doesn’t end at Phase 3
While the speed at which these vaccines are progressing is unrivalled in humanity’s vaccinology history, experts warn we still have a long way to go to achieve a safe and effective vaccine.
Although clinical trials can show that a vaccine is safe and effective among tens of thousands and even hundreds of thousands of people, it’s not possible for these trials to encapsulate every possible side effect that could occur among certain people or after an extended period of time.
That’s why, even after a vaccine has been rolled out, it’s critical to monitor the safety and efficacy of vaccines, says Naor Bar Zeev, deputy director of the International Vaccine Access Center at the John Hopkins Bloomberg School of Public Health in the US.
“There is never certainty,” Bar Zeev told DW. This ongoing surveillance is sometimes referred to as ‘Phase 4’ in clinical trials — and it can take many years.
“I think the public expectation is that once a vaccine is available we go back to the way things were — we stop wearing masks, we stop social distancing. But that is a misjudgement of what a vaccine — at least in the initial phase — can do,” Bar Zeev said, emphasizing that the delivery and ongoing evaluation of widespread vaccine deployment is a lengthy process.
